To begin the Regulatory Compliance Associates scoping process these days, please enter your details in the blue kind underneath and click the submit button at The underside in the webpage.
Also, simply because they were not used to remaining audited, they explained many things that weren't so strategic to explain.
Is the quantity of sample collected ample for Assessment and reserve in the event retesting or verification is required?
Variations in Performing procedures can be challenging to convey about. Involving the pertinent men and women within the audit will make utilizing any variations less difficult. Possession in the audit is vital. If modify in practice is needed, participants should have the ability to see why or motivation to alter won't be current.
It discusses scheduling, conducting, and reporting on audits. The real key targets of audits are to be sure excellent, assess efficiency of good quality assurance programs, and permit well timed correction of any issues. Audits aid Establish self-confidence in top quality administration techniques and detect areas for advancement.
§211.sixty five(b) Are layout and working safeguards taken to make certain lubricants or coolants or other operating substances never occur into contact with drug factors or finished products?
” Audit trail functionalities have to be enabled and locked whatsoever time and it ought to not possible to deactivate performance”
Compliance report in conjunction with all supporting files shall be submitted to QA in thirty calendar times from your date of receipt of audit observation report.
The objectives of auditing are to determine conformity and effectiveness of high-quality devices. Audits are crucial for compliance, trouble detection, and examining Command units. The doc outlines standard audit treatments, classifications, types of auditors, along with the ten move auditing procedure Employed in the pharmaceutical industry.
To execute an audit and compare recent follow into the regular established, facts (information and facts) need to be gathered.It is necessary to gather ideal information only and to keep facts selection so simple as achievable.
This guidebook has long been current from earlier Focus on audit performed from the Royal PharmaceuticalSociety and should help pharmacists apply the audit specifications of the new pharmacy contract released in England and Wales on 1 April 2005.
One more tactic can be not to start out the audit in one of the most reasonable get. By beginning website in yet another way, you can begin with the topic you discover much more important. If you start, for instance, from the warehouse, and that is a considerably less significant place, you would possibly lose a lot of time there.
I do not forget that from time to time they welcomed me with bouquets, sang their countrywide tune to me, and experienced their nation flag out. website And it absolutely was an occasion for them that a lot of people from a Western company came to audit their plant.
Is basic safety schooling documented in a very quickly retrievable fashion that states the identify of the worker, the kind of training, the date from the coaching, along with the title in the coach and also the signature from the coach plus the participant?
 Judge Reinhold Then & Now!
Judge Reinhold Then & Now! Michael Fishman Then & Now!
Michael Fishman Then & Now! Hailie Jade Scott Mathers Then & Now!
Hailie Jade Scott Mathers Then & Now! Monica Lewinsky Then & Now!
Monica Lewinsky Then & Now!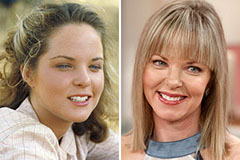 Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now!